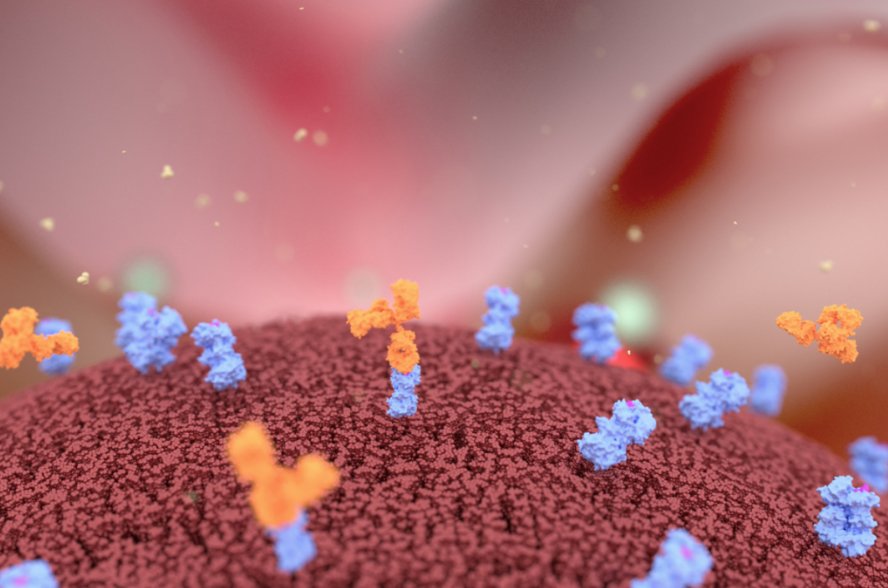
Canonical antibodies are formed with two identical polypeptide heavy chains and light chains. They consist of antigen-binding (Fab’) regions for specificity and the Fc domain for effector function and structural stability. Antibody therapies, checkpoint blockers like PD-L1, PD-1, and CTLA-4, can transform cancer treatment. Immunostimulatory antibodies activate immune cells by targeting the co-stimulatory receptors like CD40, 4-1BB, OX40, and CD27 from the tumor necrosis factor receptor superfamily (TNFRSF). Early clinical trial antibodies like Utomilumab, Urelumab, and 4-1BB mAb have raised concerns regarding toxicity and efficacy.
This study is focused on determining how the structural modifications, specifically disulfide engineering, inside and outside the human IgG2 hinge region (hIgG2) antibody. These modifications can enhance conformational rigidity, increase immunostimulatory activity, and develop more effective and potent antibody treatments for cancer immunotherapy. This study analyzes the functional and structural efficiency of the therapeutic antibodies with a focus on how modification to the hinge region, especially in the human hIgG2, affects it. It can improve immunostimulatory activity against cancer.
This study followed the ethical guidelines of the University of Southampton by using blood cones from healthy adult donors with informed consent. Antibodies were produced by using site-directed mutagenesis and protein production in the ExpiCHO-S cells. It is purified by using size exclusion chromatography and protein A affinity chromatography. The antibodies were low in aggregation and endotoxin content. F(ab’)2 fragments were produced and purified, and their purity was analyzed. Immunostimulatory activity was determined by NF-κB activation by using primary human B cells and Jurkat cells.